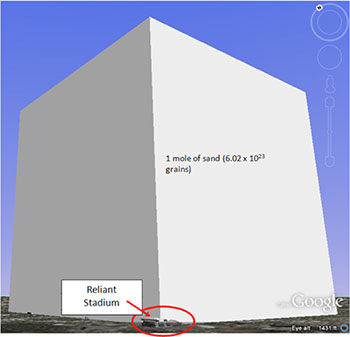
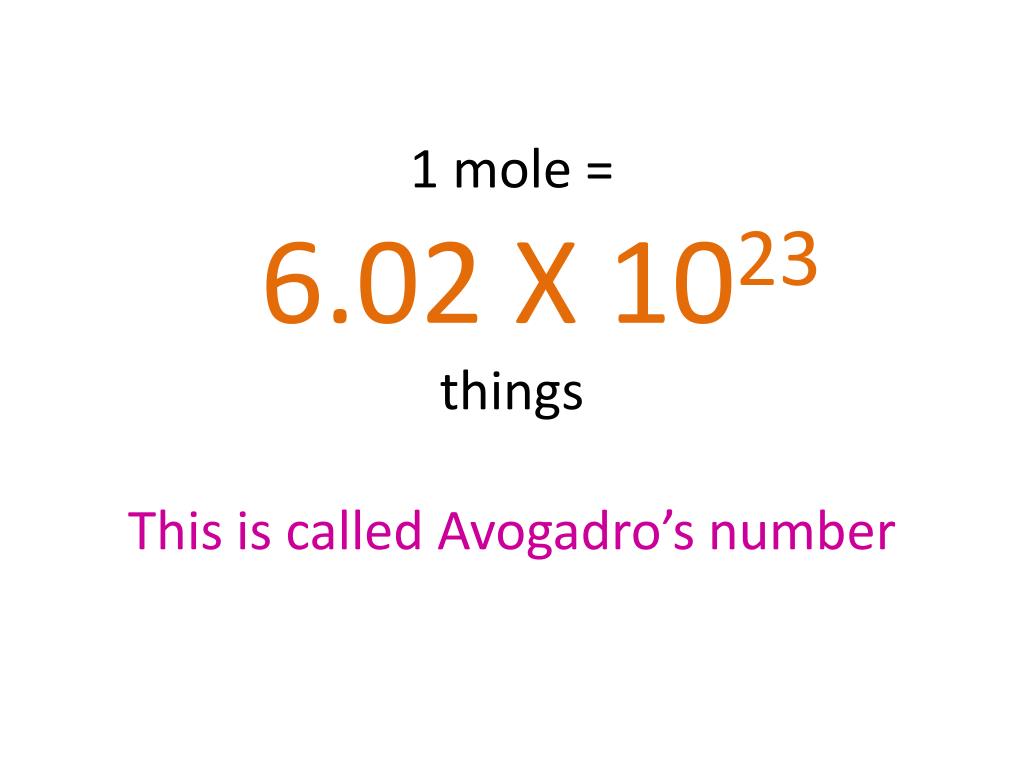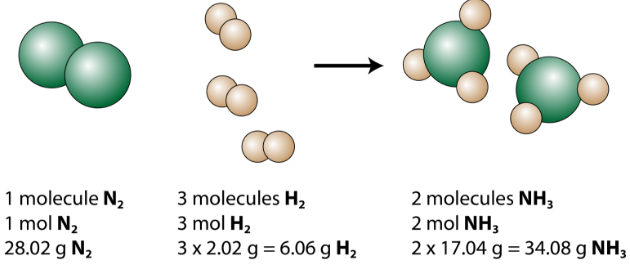The Mole 1 dozen = 1 century= 1 pair = 1 mole = x There are exactly 12 grams of carbon-12 in one mole of carbon ppt download

Analysis of Two Definitions of the Mole That Are in Simultaneous Use, and Their Surprising Consequences | Journal of Chemical Education