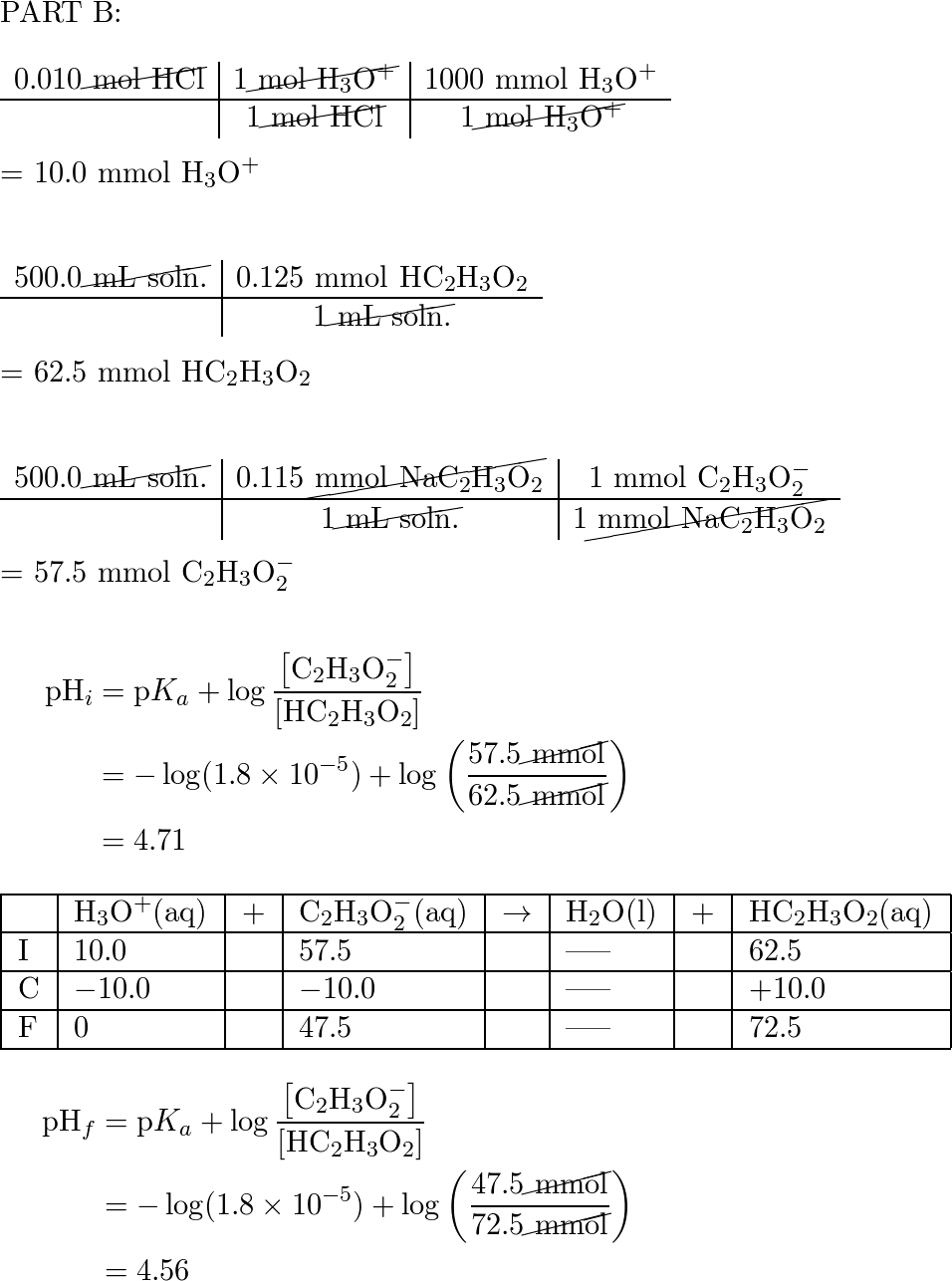
How many gram moles of HCl will be required to prepare one litre of a buffer solution (containing NaCN and HCN ) of pH 8.5 using 0.10g formula mass of NaCN ?

10 moles of HCL is added to excess to magnesium and forms 4 moles of hydrogen gas then Percentage - Brainly.in

Hydrochloric Acid, c(HCl)=0.1 mol/L (0.1N) Titripur , MilliporeSigma, Quantity: 1 L | Fisher Scientific
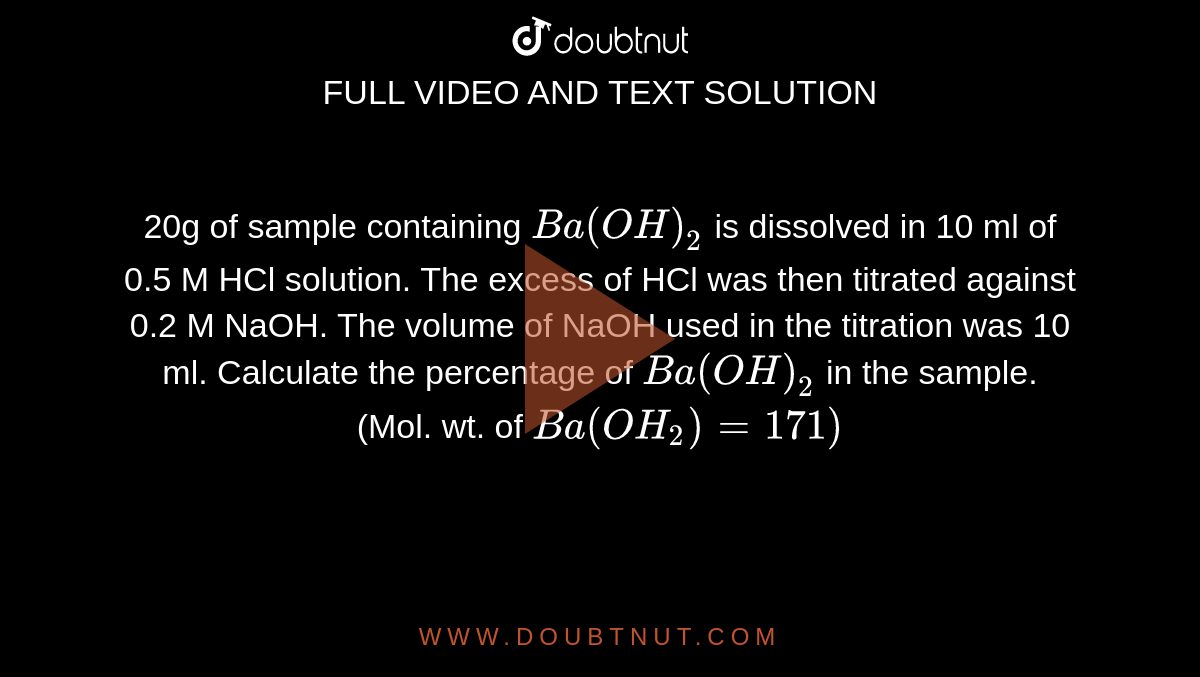
20g of sample containing Ba(OH)(2) is dissolved in 10 ml of 0.5 M HCl solution. The excess of HCl was then titrated against 0.2 M NaOH. The volume of NaOH used in
Assuming that: Part I (molarity of HCl by standardization): Part II (determination of sodium carbonate in unknown) Solution: Mas