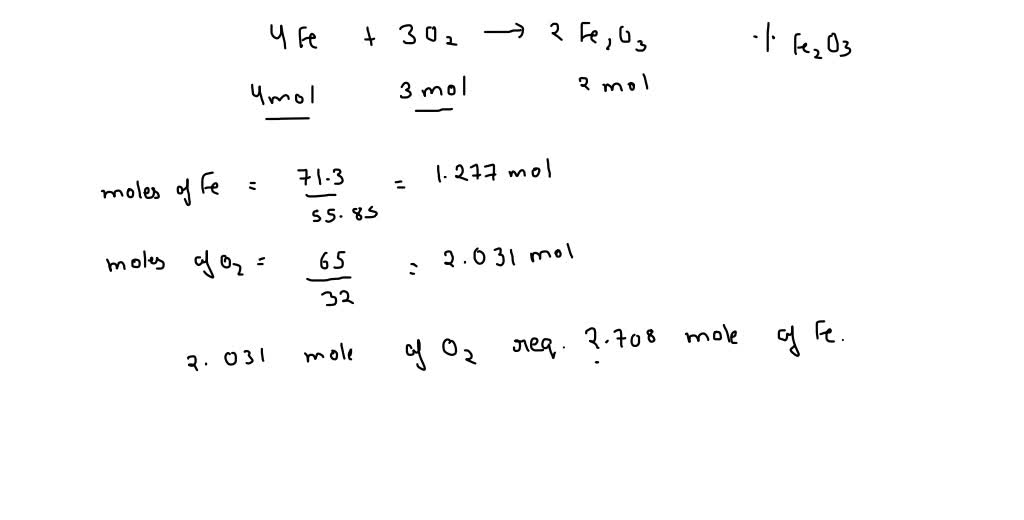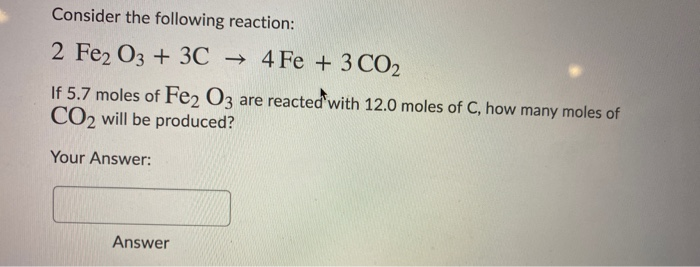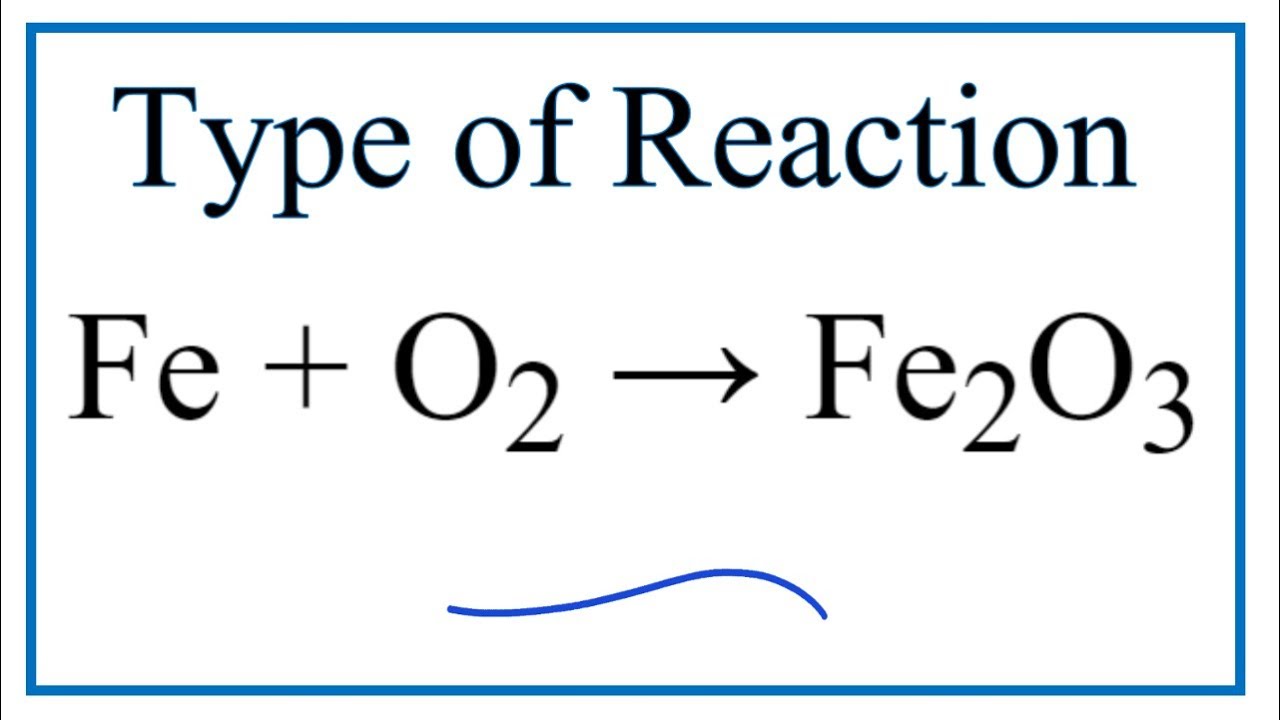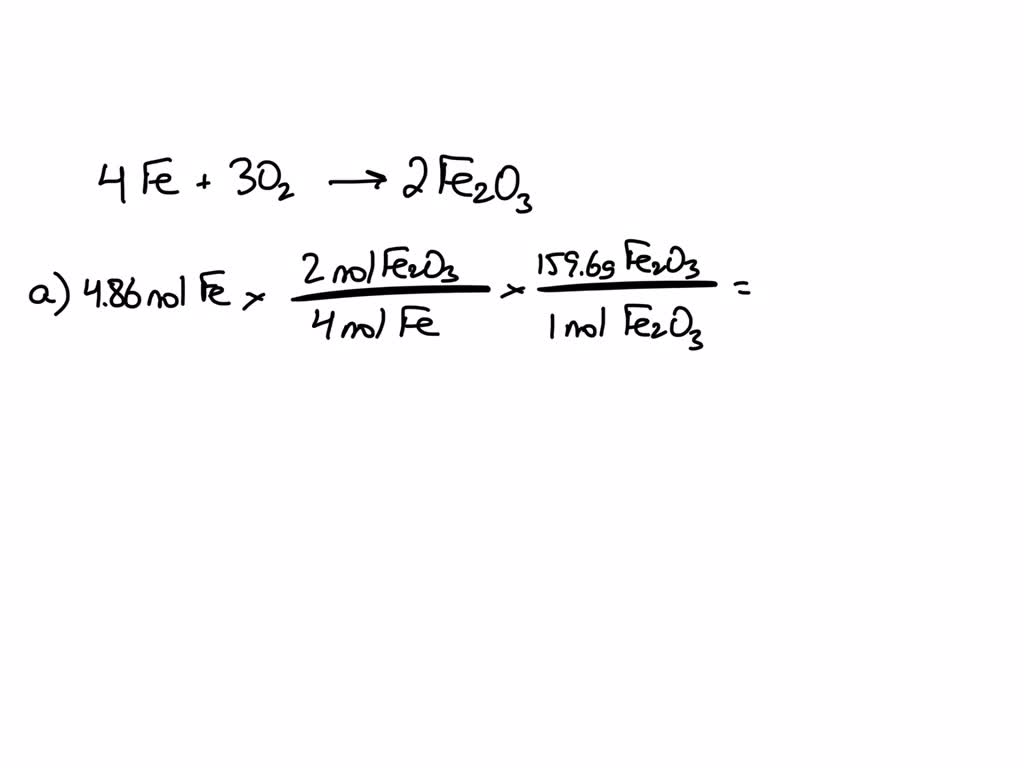
SOLVED: Given the reaction 4Fe + 3O2= 2Fe2O3 a. How many grams of Fe2O3 will be formed from 4.86 moles Fe reacting with sufficient oxygen gas? b. How many grams of Fe
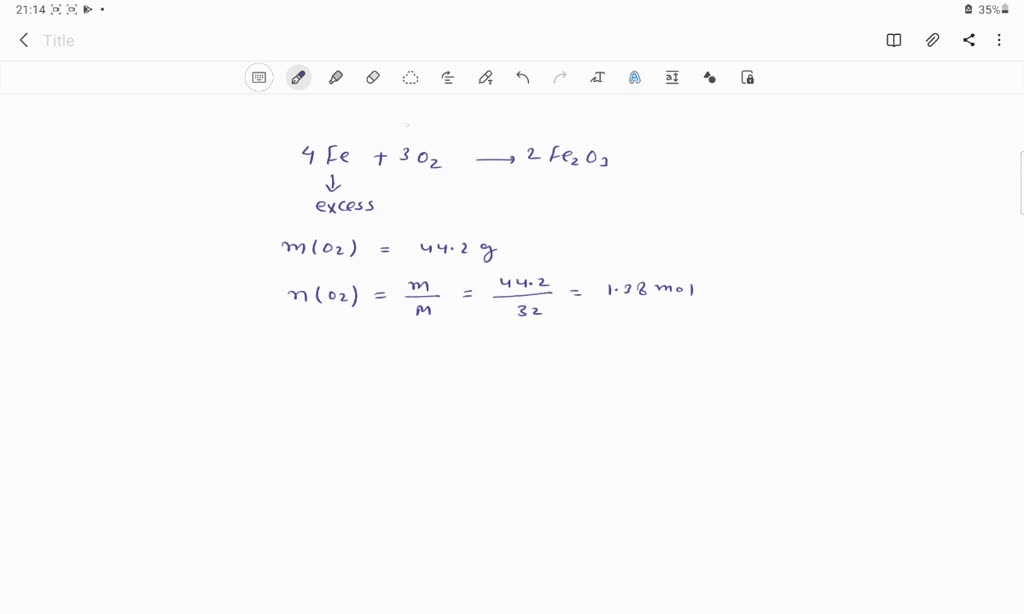
SOLVED: 4Fe + 3O2 2Fe2O3 If 44.2 grams of oxygen react with an excess of iron, what mass of Fe2O3 can form? (Fe2O3= 159.70 g/mol) a. 147 g b. 331 g c. 294 g d. 221 g

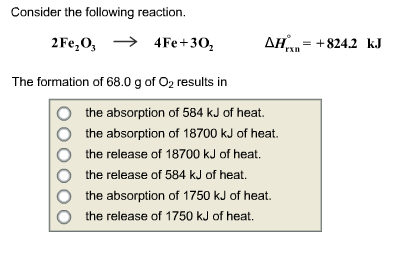
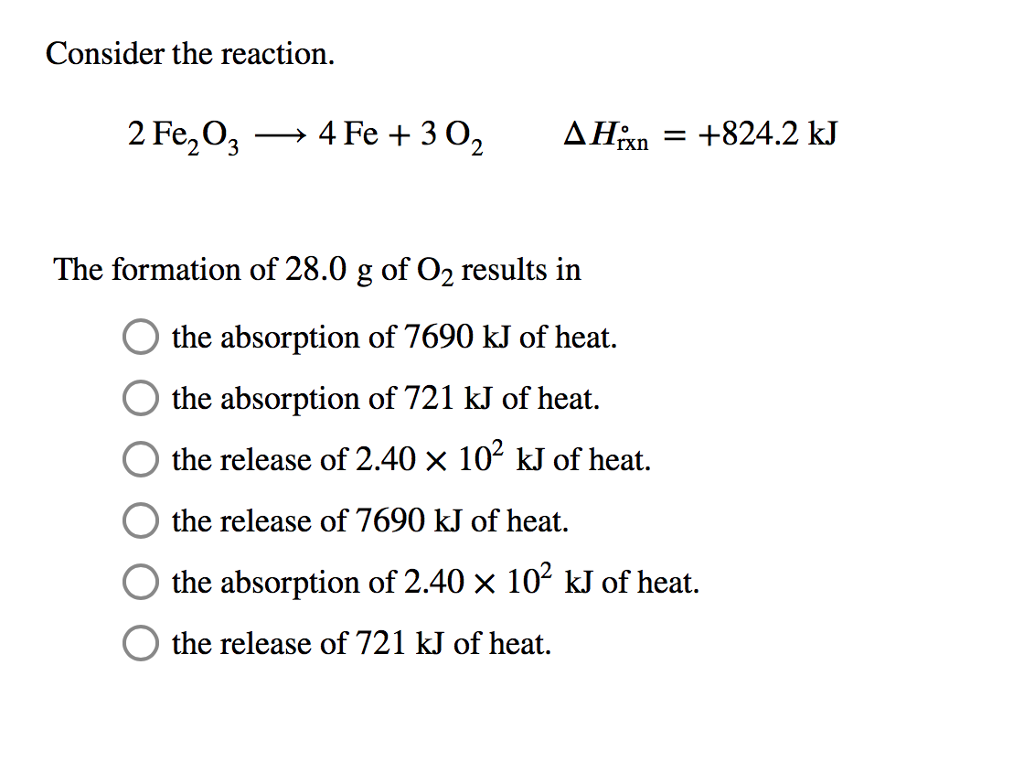
SOLVED: For the oxidation of iron to form iron(III) oxide: 4Fe(s) + 3O2(g) ⇌ 2Fe2O3(s) ΔS° = –549.73 J/K at 298 K The enthalpy of formation of Fe2O3(s) is –824.2 kJ/mol. What
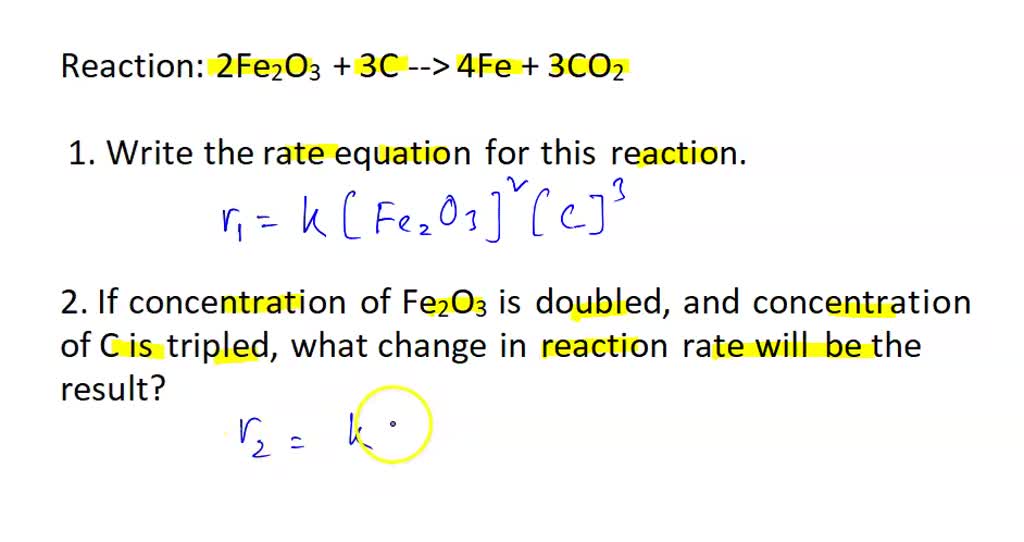
SOLVED: Reaction: 2Fe2O3 + 3C –> 4Fe + 3CO2 1. Write the equation for this reaction. 2. If concentration of Fe2O3 is doubled, and concentration of C is tripled, what change in