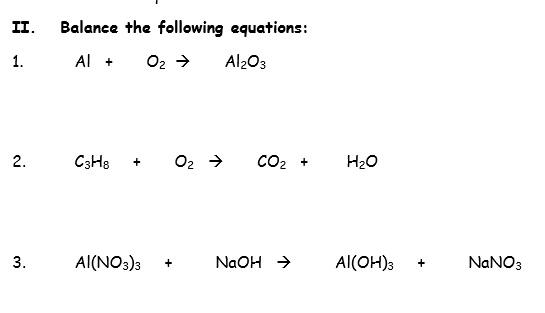
Balance the following equation and identify what type of reaction it is. Al( OH)3 arrow Al2O3 + H2O | Homework.Study.com

How to Balance Al(OH)3= Al2O3+ H2O|Chemical equation Al(OH)3=Al2O3+H2O|Al(OH )3=Al2O3+H2O Balance - YouTube

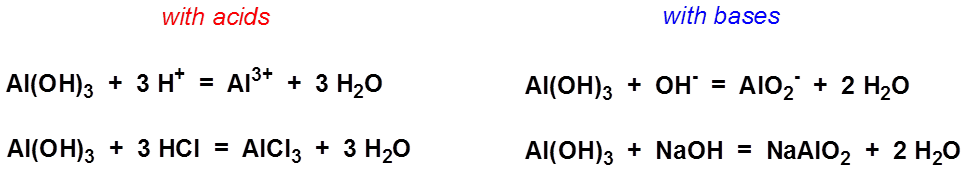
6с Напишите уравнения реакций следующих превращений: Al → AlCl3 → Al(OH)3 → Al2O3 → NaAlO2 → Al2(SO4)3 → Al(ОН)3 → AlCl3 →NaAlO2 Реакции, идущие с участием электролитов, запишите в ионной форме. Первую реакцию рассм

0.078 g Al(OH)(3). is dehydrated to Al(2)O(3). The Al(2)O(3) so obtained reacted with 6 milli-equivalent of HCl. The equivalent of AlCl(3) produced during the reaction are:
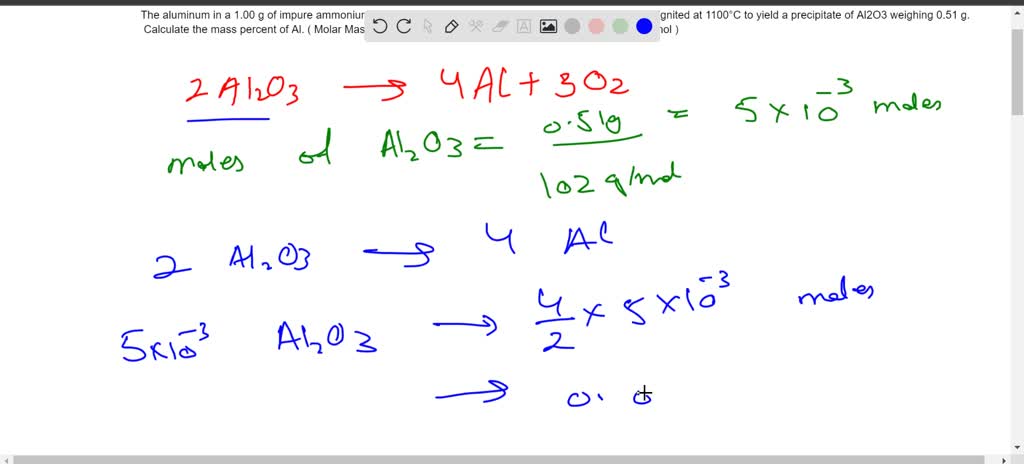
SOLVED: The aluminum in a 1.00 g of impure ammonium aluminum sulfate sample was precipitated as Al(OH)3 and ignited at 1100°C to yield a precipitate of Al2O3 weighing 0.51 g. Calculate the

Addition of Al(OH)3 versus AlO(OH) nanoparticles on the optical, thermo-mechanical and heat/oxygen transmission properties of microfibrillated cellulose films | SpringerLink