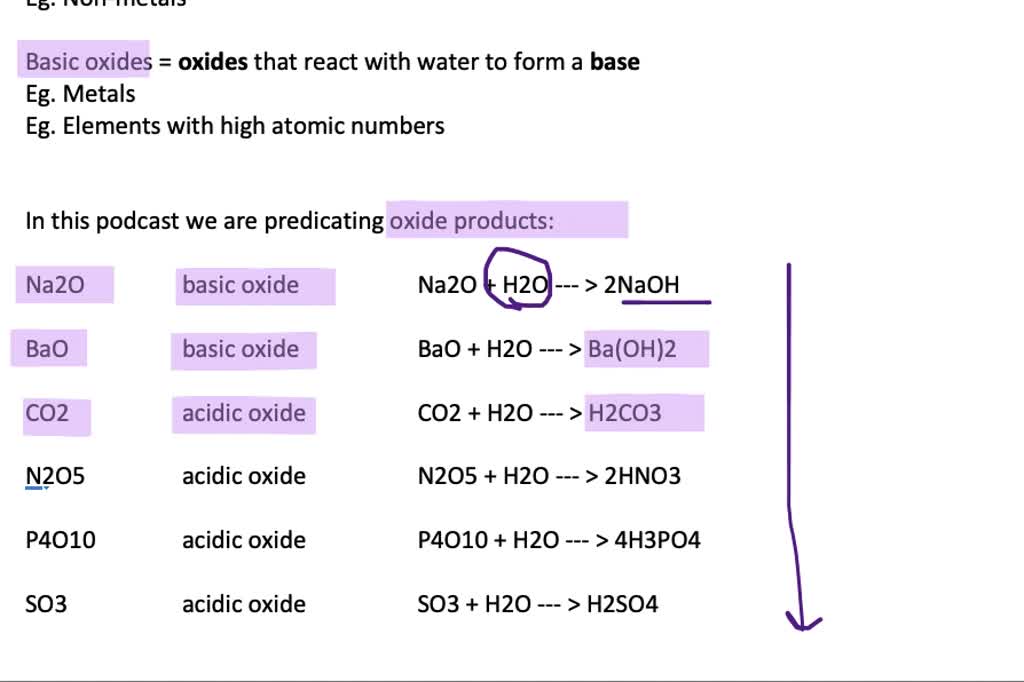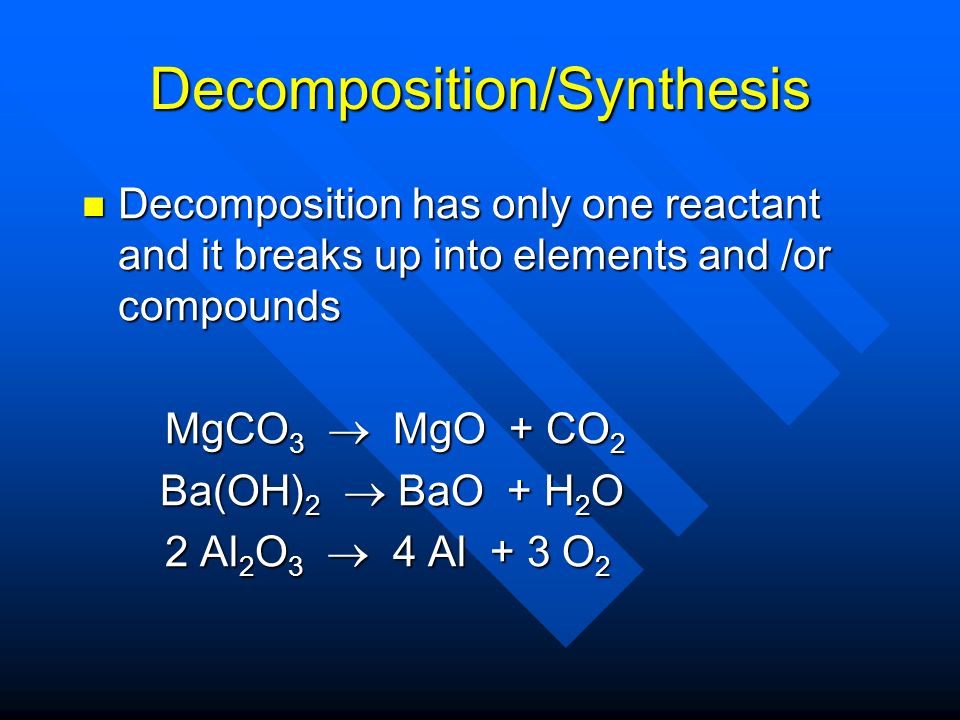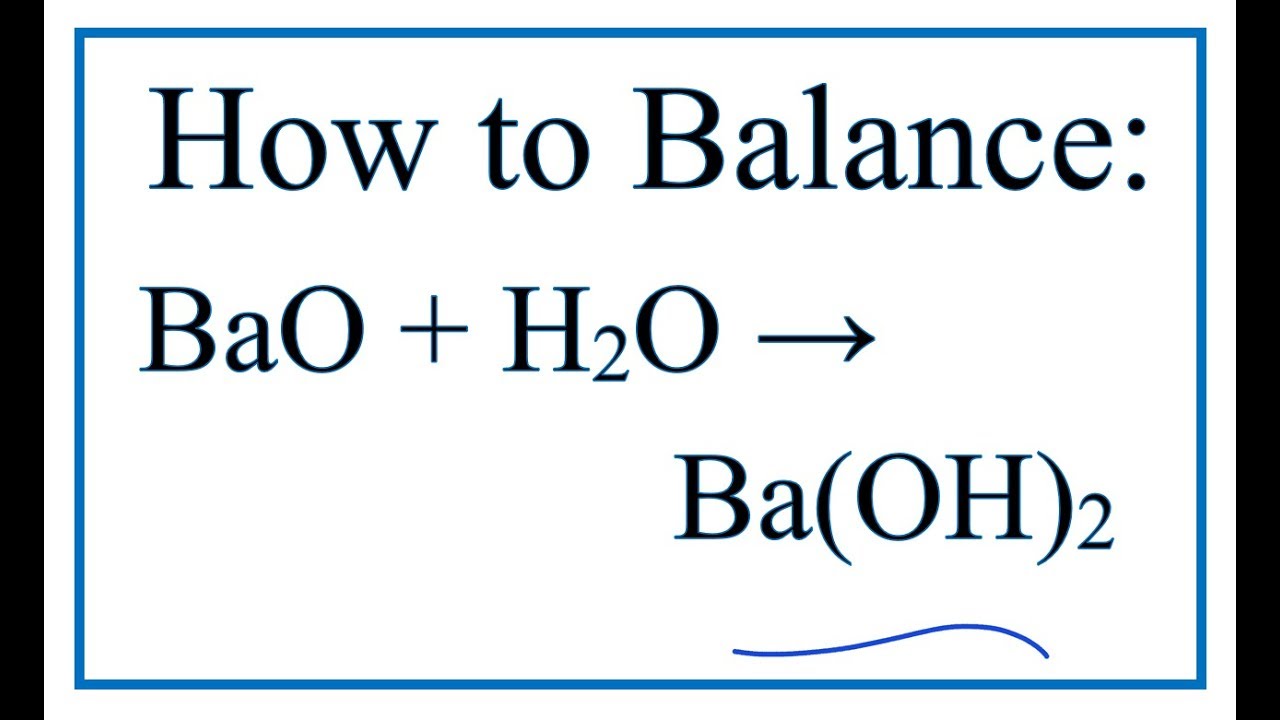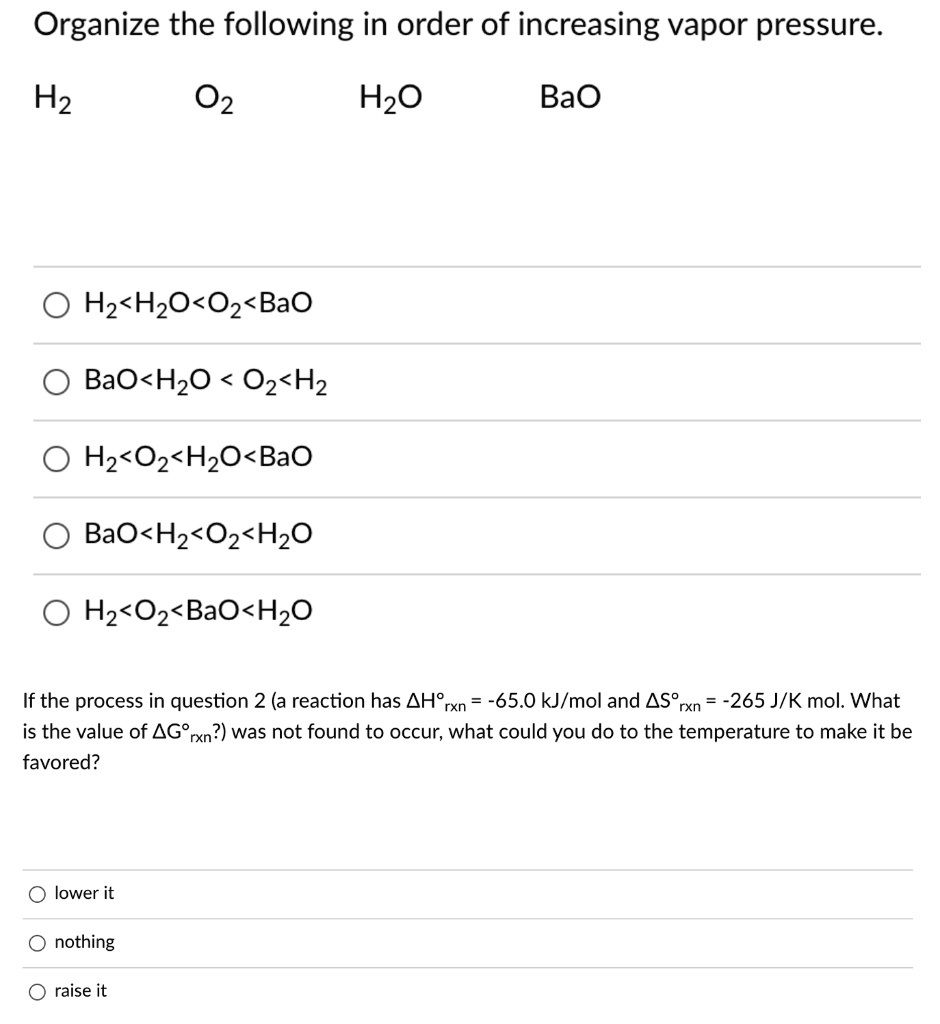
SOLVED: Organize the following in order of increasing vapor pressure: H2 02 HzO BaO H2<H2O<O2<BaO BaO<HzO < O2<Hz H2<02<HzO<BaO BaO<H2<02<HzO H2<02<BaO<HzO If the process in question 2 (a reaction has AHS rxn = -

SOLVED: Total marks: 50.0/4-14. Note: Round all atomic weights to decimal places- Problem 1. (5 Points) Write balanced equation that predict the reactions of solid zinc with the following: a) HCI b)
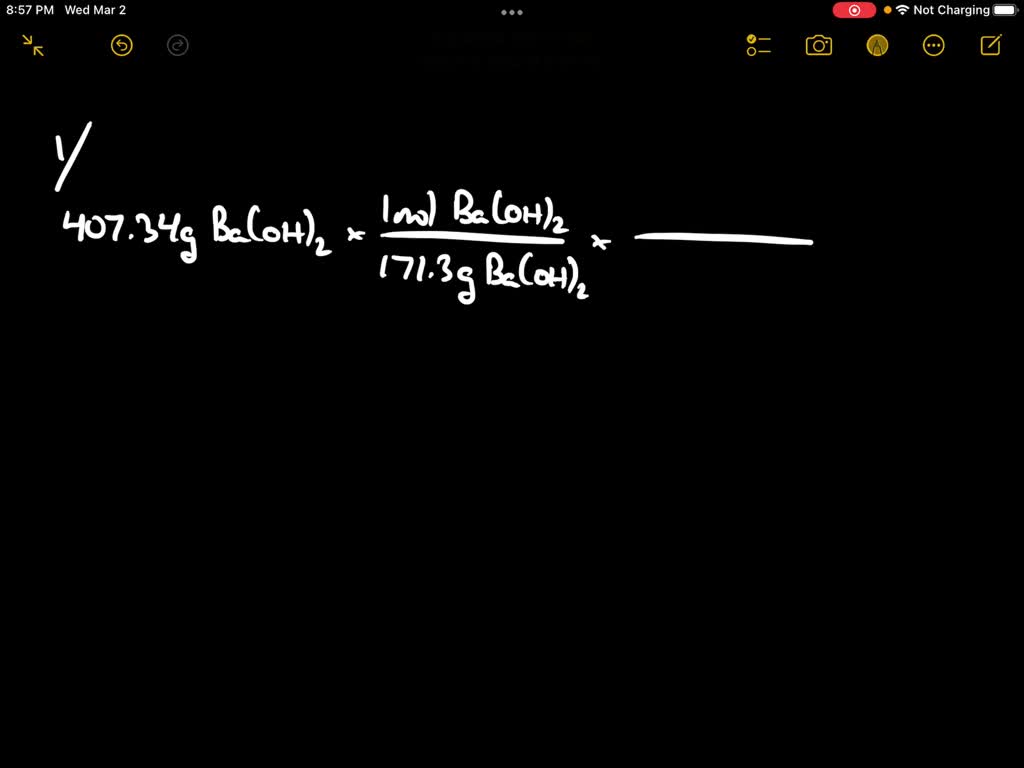
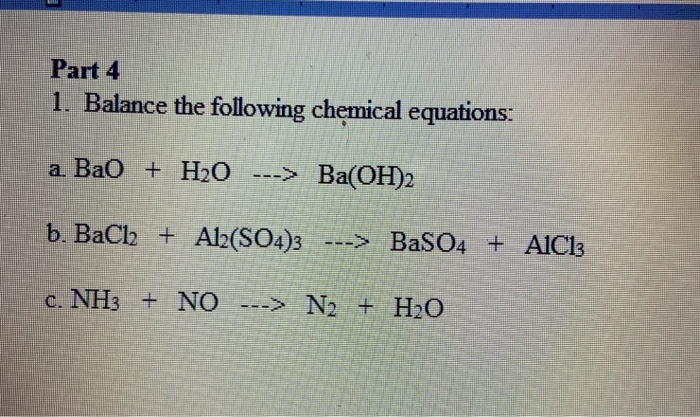
SOLVED: 1. BaO + H2O â†' Ba(OH)2 Given that you have: 407.34 grams Ba(OH)2 produced How many particles of BaO reacted? 2. For this reaction: K3PO4 + Al(NO3)3 â†' 3 KNO3 +