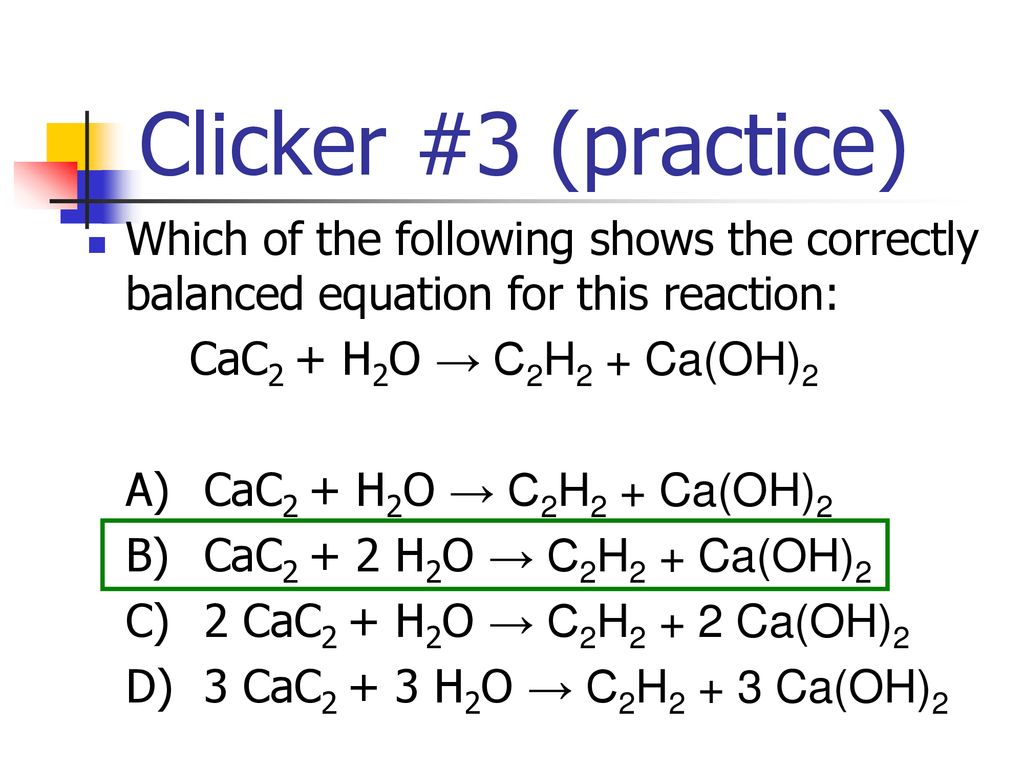
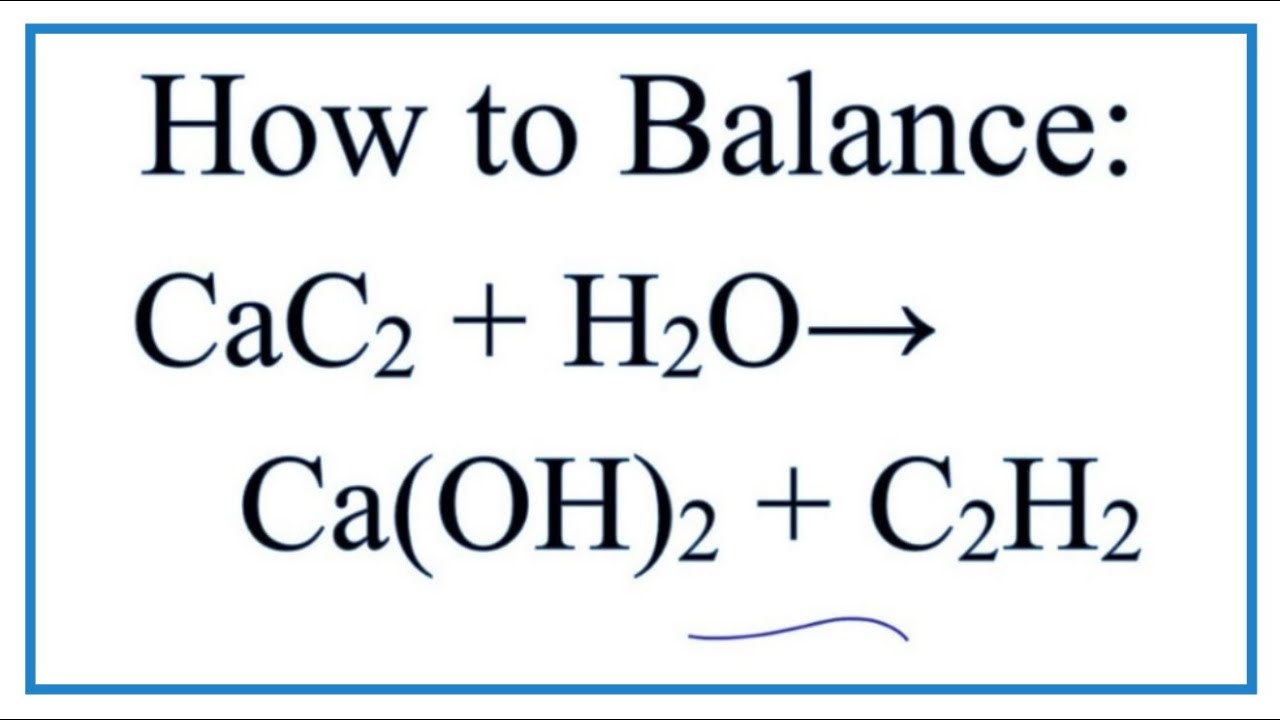
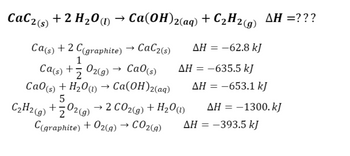Calcium carbide, CaC2, reacts with water to form ethyne, C2H2, and calcium hydroxide. The equation for the reaction is shown. CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH) 2(s), which volume of ethyne
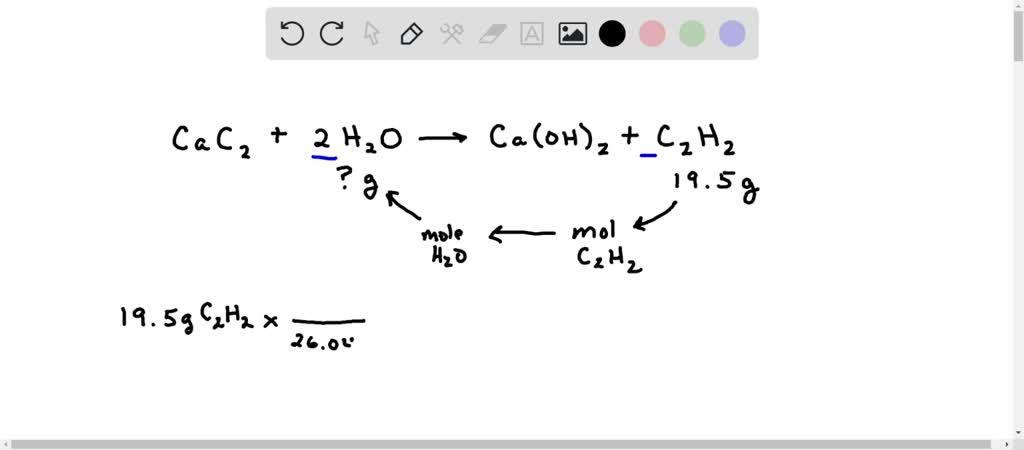
SOLVED: Calcium carbide (CaC2) reacts with water to form acetylene (C2H2): CaC2 (s) + 2 H2O (g) → Ca(OH)2 (s) + C2H2 (g) How many grams of water are required to produce
CaC2 reacts with H2O and gives X. X reacts with Cu2Cl2 in the presence of NH4OH and give Y. What is Y, and how? - Quora
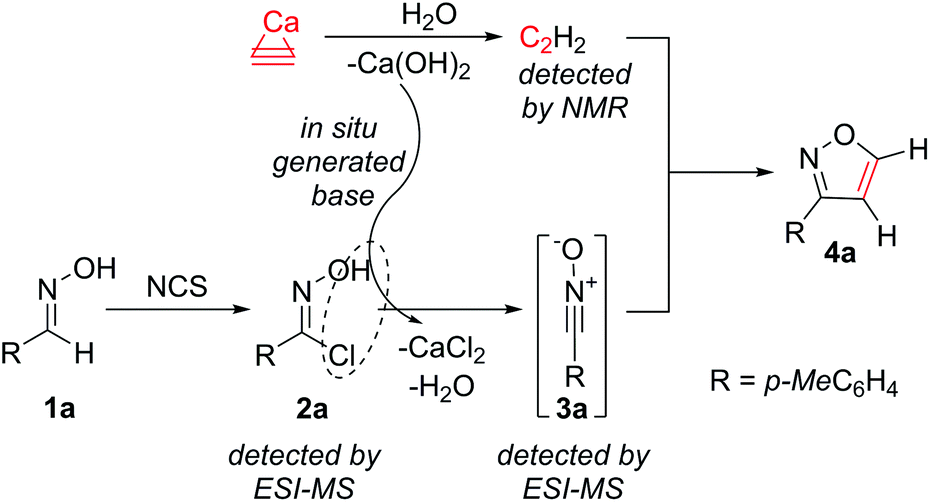
Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C7QO00705A
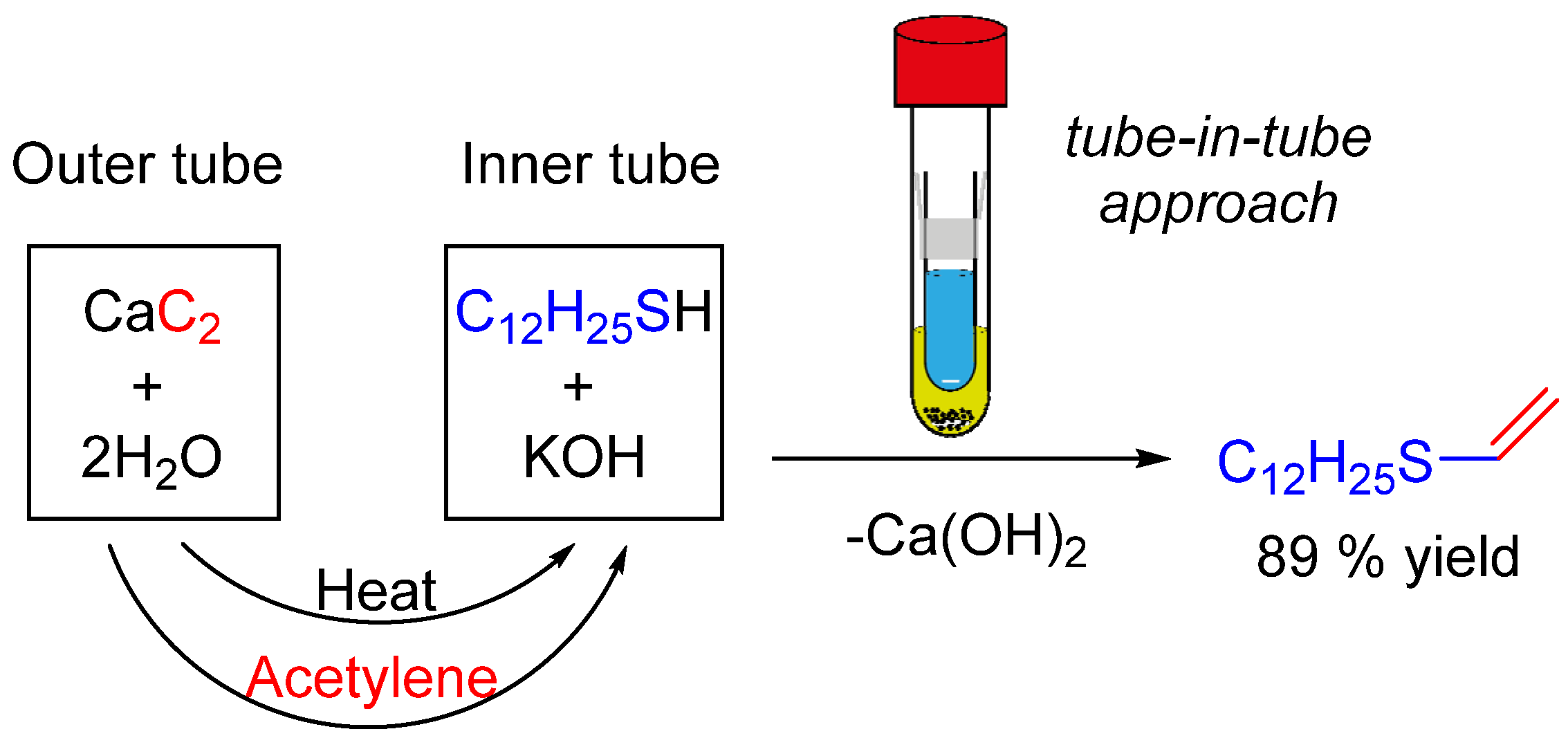
IJMS | Free Full-Text | Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes

Fluoride-Assisted Activation of Calcium Carbide: A Simple Method for the Ethynylation of Aldehydes and Ketones | Organic Letters