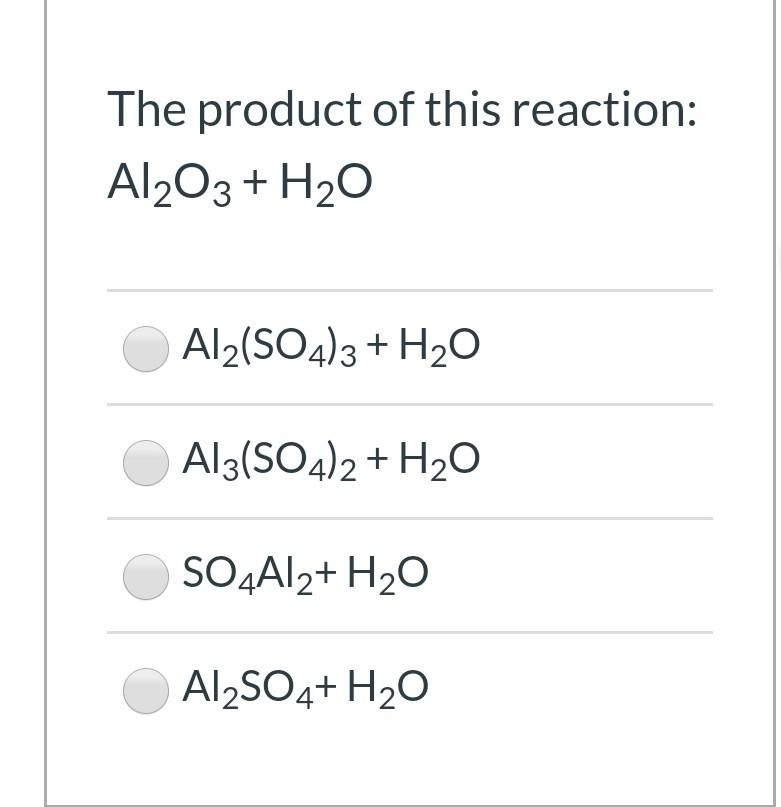
H2O+H2SO4=H3O+SO4 Balance the equation. h2o+h2so4=h3o+so4 water and Sulfuric acid reacts to form - YouTube

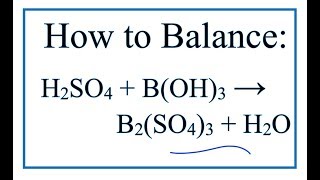
Balance the reaction and explain what type of the reaction is: H_2SO_4 + B(OH)_3 to B_2(SO_4)_3+H_2O | Homework.Study.com
, RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry RE(SO4)[B(OH)4](H2O), RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.7b02317/asset/images/large/ic-2017-02317c_0008.jpeg)
RE(SO4)[B(OH)4](H2O), RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry

Fe(OH)3 + H2SO4(dil.) = Fe2(SO4)3 + H2O Is it a balanved equation? what are the number of atoms of the - Brainly.in






![Thermal effects in [CsEu(H2O)3(SO4)2]·H2O. | Download Scientific Diagram Thermal effects in [CsEu(H2O)3(SO4)2]·H2O. | Download Scientific Diagram](https://www.researchgate.net/profile/Aleksandr-Oreshonkov/publication/354150483/figure/tbl1/AS:1061074447114240@1629991270579/Thermal-effects-in-CsEuH2O3SO42H2O_Q320.jpg)