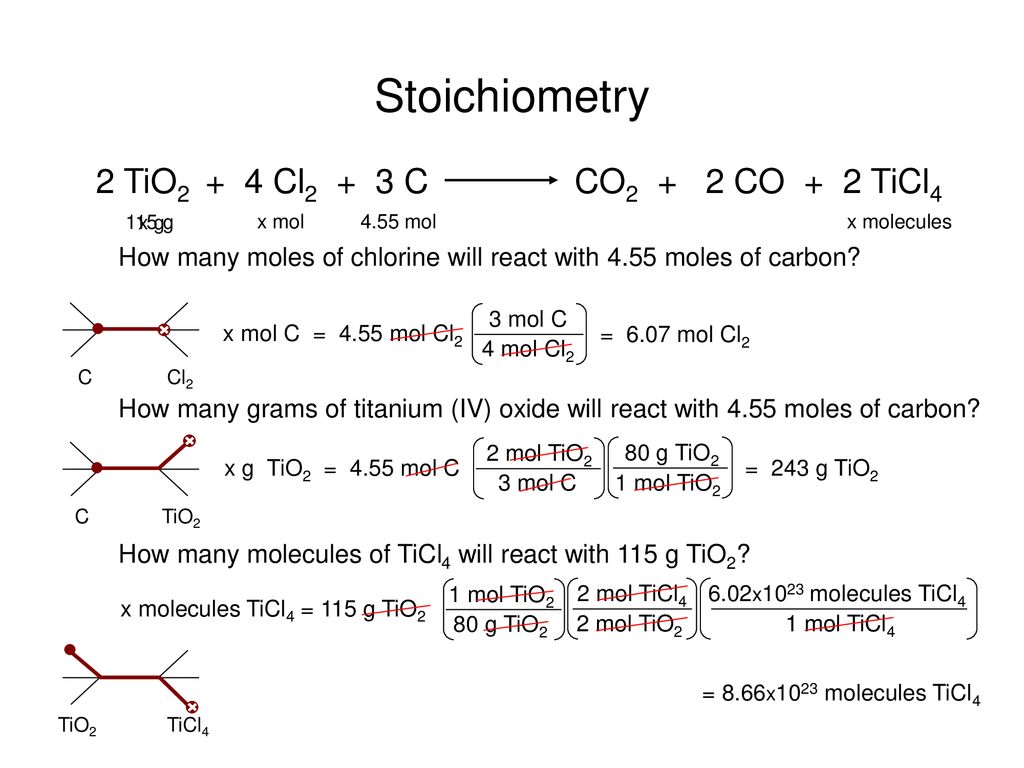
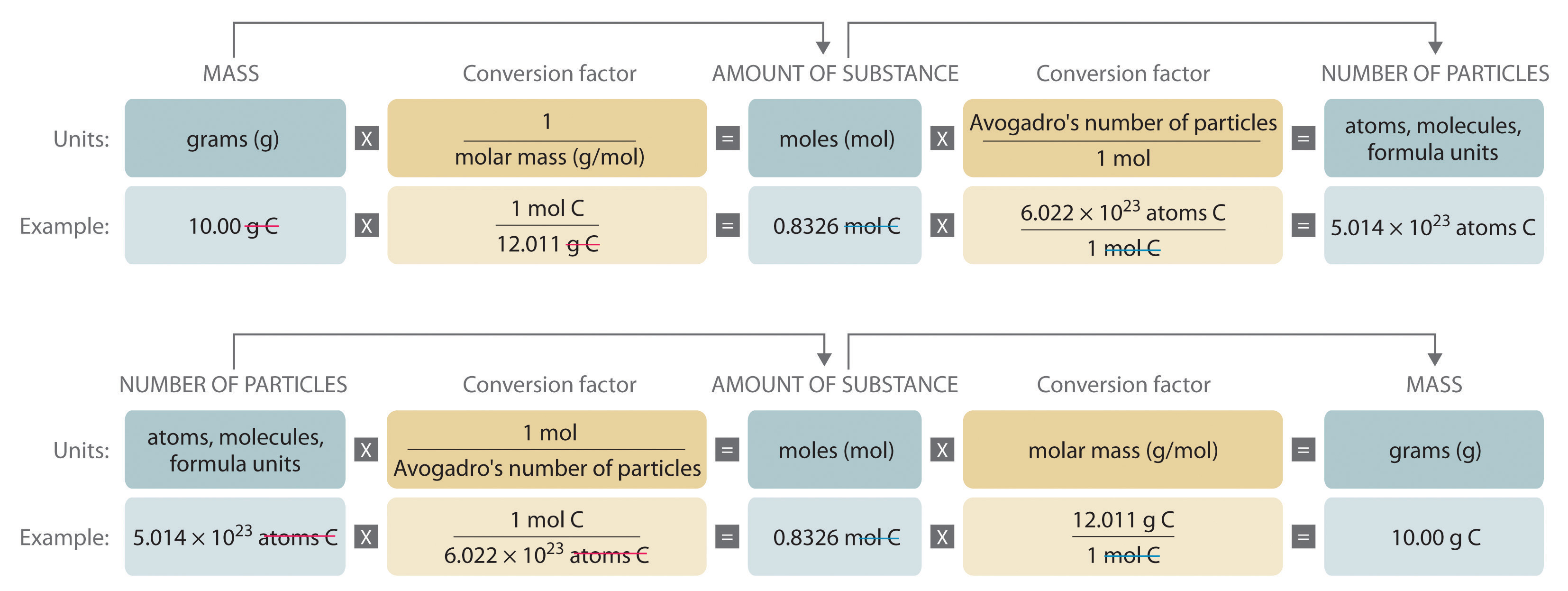
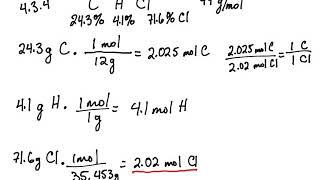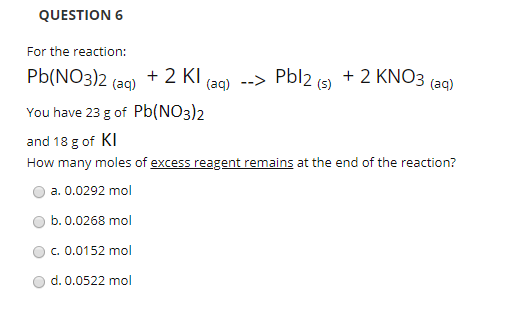141 Mole Worksheet Key - Mass and Moles Worksheet How many moles in 34 g of sulfur? 34 푔 푆 × 1 푚표푙 푆 - Studocu

Draw the complete molecular orbital diagram for C^+_2 (form the molecular orbital diagram from the combination of a neutral C and a cationic C^+). | Homework.Study.com
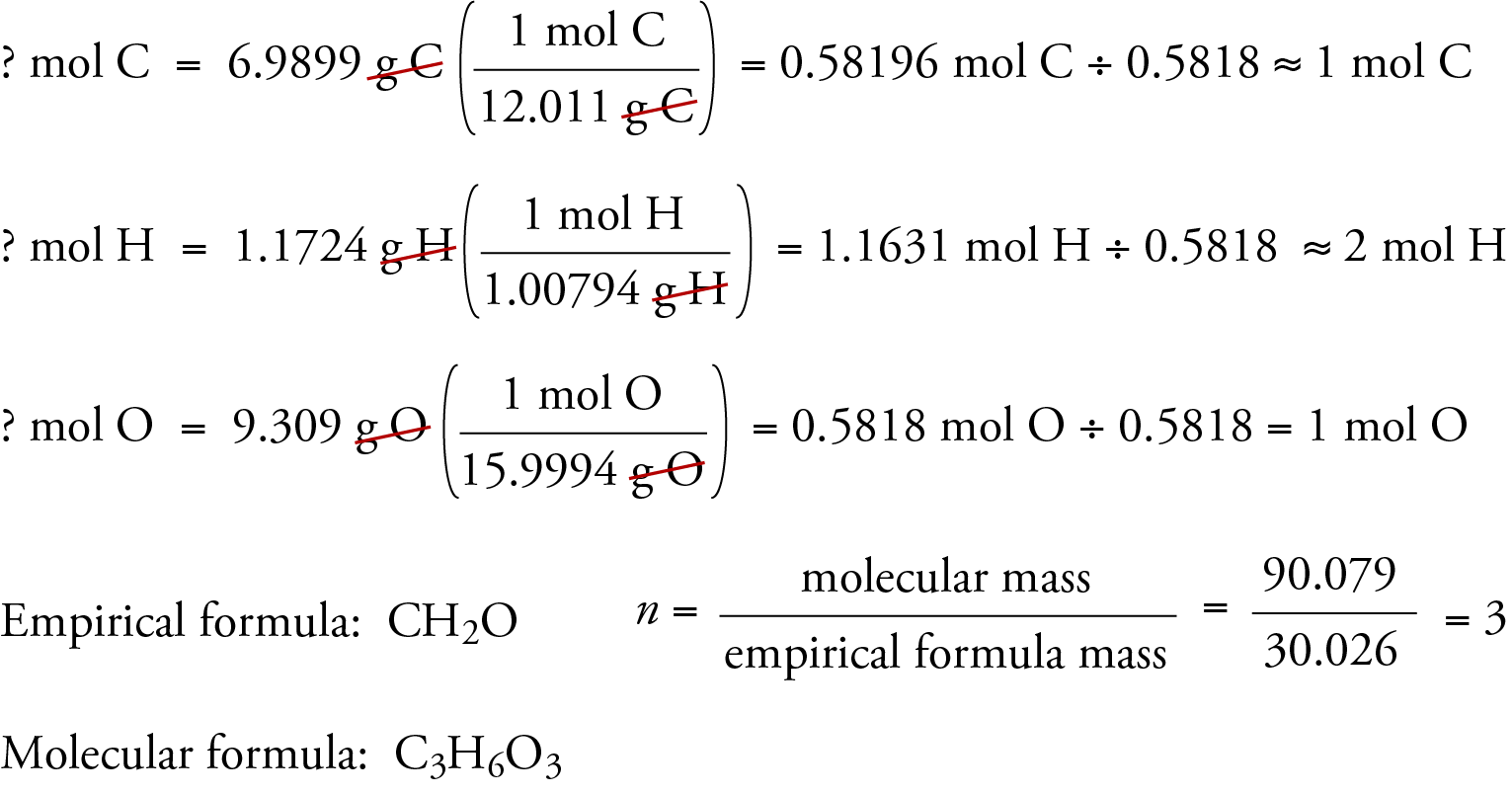
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are