
Measurement and Modeling of the Solubility of NH4VO3 in the Na2HPO4–H2O and (NH4)2HPO4–H2O Systems | Journal of Chemical & Engineering Data

Equilibrium constants is given (in atm) for the following reaction 0^∘C : Na2HPO4. 12H2O(s) Na2HPO4. 7H2O(s) + 5H2O(g) ; Kp = 2.43 × 10^-13 The vapour pressure of water at 0^∘C is

Comparative Study of Sodium Phosphate and Sodium Sulfate in Aqueous Solutions at (298.15 to 353.15) K | Journal of Chemical & Engineering Data
![Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 1kg) [CN04-1KG] - $36.00 : Bioland Scientific, for Your Research Needs Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 1kg) [CN04-1KG] - $36.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/NaH2PO4s%201KG.jpg)
Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 1kg) [CN04-1KG] - $36.00 : Bioland Scientific, for Your Research Needs
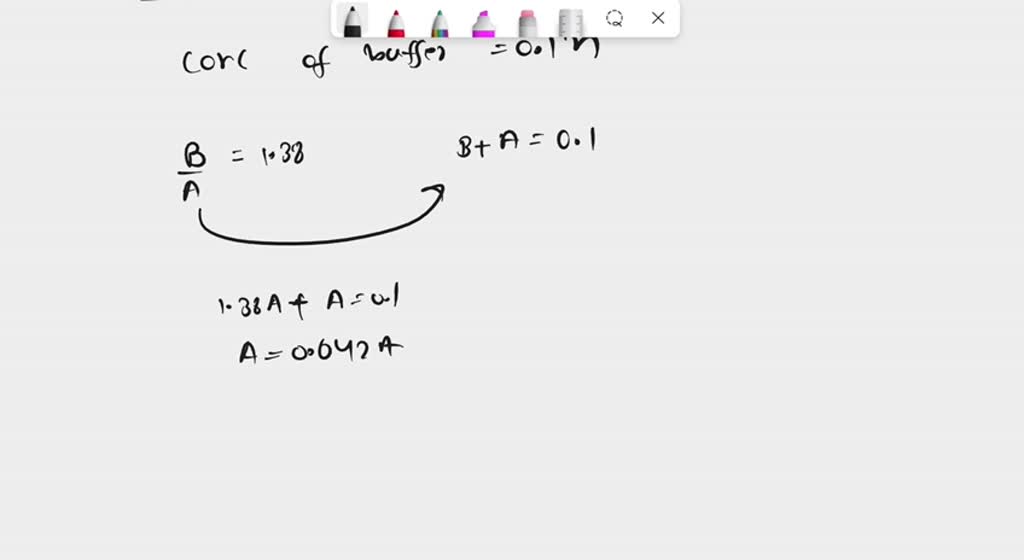
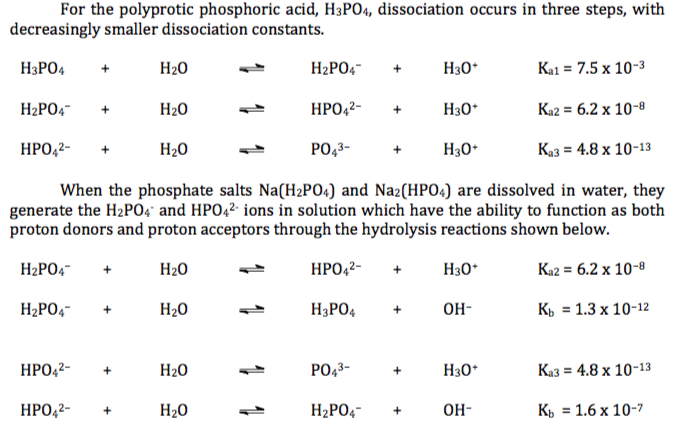
SOLVED: 1. How many g of Na2HPO4 and NaH2PO4 2H2O would you need to prepare 1L of 0.1M sodium phosphate buffer pH 7.0? (Hint= use the Henderson-Hasselbalch equation) Express your answer to

SOLVED: how do I make pH 9 from Na2HPO4.2H2O and NaH2PO4.H2O in 1000 ml, how much mass do I have to take from each

For the equilibrium SrCl2· 6H2O(s) SrCl2· 2H2O(s) + 4H2O(g) the equilibrium constant Kp = 16 × 10^-12 atm^4 at 1^0 C. If one litre of air saturated with water vapour at 1^0




2MoO4%20+%20HNO3%20=%20(NH4)3(PMo12O40)%20+%20NH4NO3%20+%20NaNO3%20+%20H2O.svg)