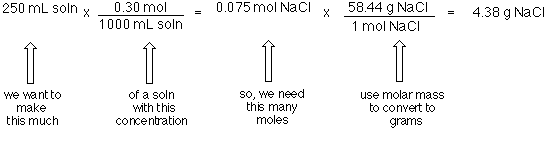Changes of phenol concentration with time for I NaCl = 1 mol kg −1 (a)... | Download Scientific Diagram

a) Digital image showing water, NaCl (20 wt%), NaOH (1 mol·L −1 ) and... | Download Scientific Diagram

The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download

The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download
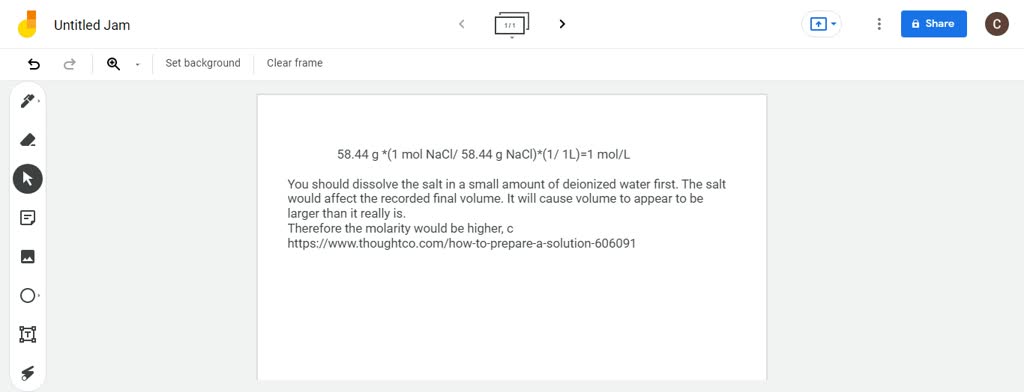
SOLVED:You prepared a NaCl solution by adding 58.44 g of NaCl to a 1 -L volumetric flask and then adding water to dissolve it. When finished, the final volume in your flask

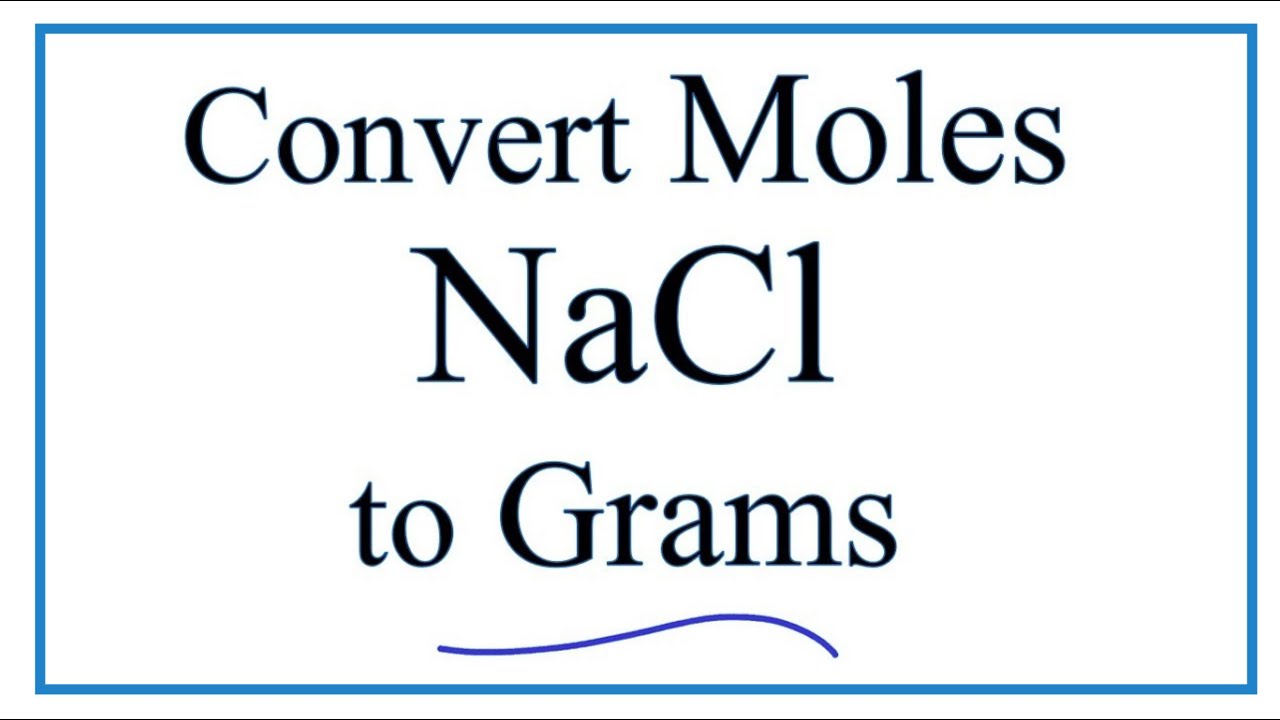
Molarity Problems – IF worksheet. 1)MW NaCl = 23 + 35 = 58g NaCl = 1 mole 2)M = moles M = x moles Liters 1.0 L 3)58 g NaCl 1 mole NaCl = 1 mole NaCl 58. - ppt download

Calculate the mass of 1 mole of each one of the following: (a) `NaCl` , (b) `CaCO_(3)` , (c ) `FeSO - YouTube

The density of `1 M` solution of `NaCl` is `1.0585 g mL^(-1)`. The molality of the solution is - YouTube