First-principles analysis of oxide-ion conduction mechanism in lanthanum silicate - Journal of Materials Chemistry (RSC Publishing)

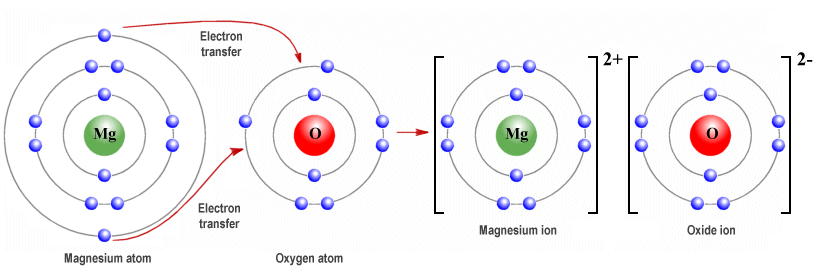
The formation of the oxide ion, O^2 - (g) , from oxygen atom requires first an exothermic and then an endothermic step as shown below: O(g) + e^ - → O^ - (

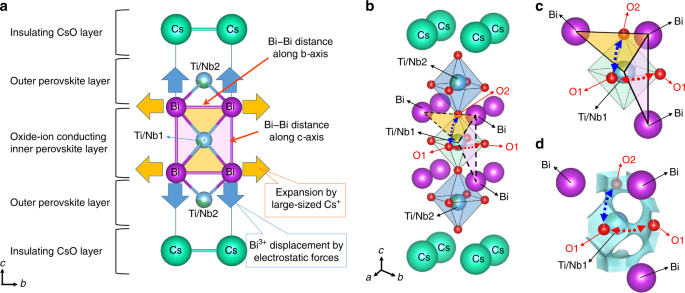
a)(b) The trajectories of oxide-ion conduction processes along the c... | Download Scientific Diagram

Theoretical Modeling of Oxide Ion Conductivity in Doped LaSrGa3O7 Melilites | The Journal of Physical Chemistry C
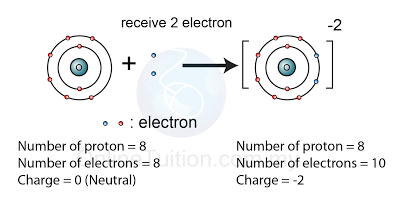
What is the difference between atoms and ions; and covalent compounds and ionic compounds? | Socratic

Chapter 20. Calculating Oxidation Numbers Each oxide ion has a charge of -2 7 oxide ions have a subtotal charge of -2 x 7 = -14 Since the formula has. - ppt download

Explain how to use Coulomb's law to calculate the energy of a zinc ion and an oxide ion at their equilibrium ion-pair separation distance. | Homework.Study.com

Ruddlesden–Popper Oxychlorides Ba3Y2O5Cl2, Sr3Sc2O5Cl2, and Sr2ScO3Cl: First Examples of Oxide-Ion-Conducting Oxychlorides | ACS Applied Energy Materials