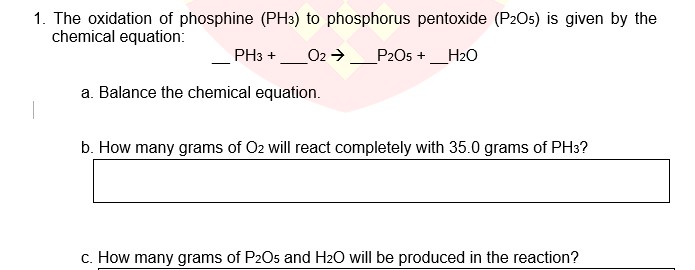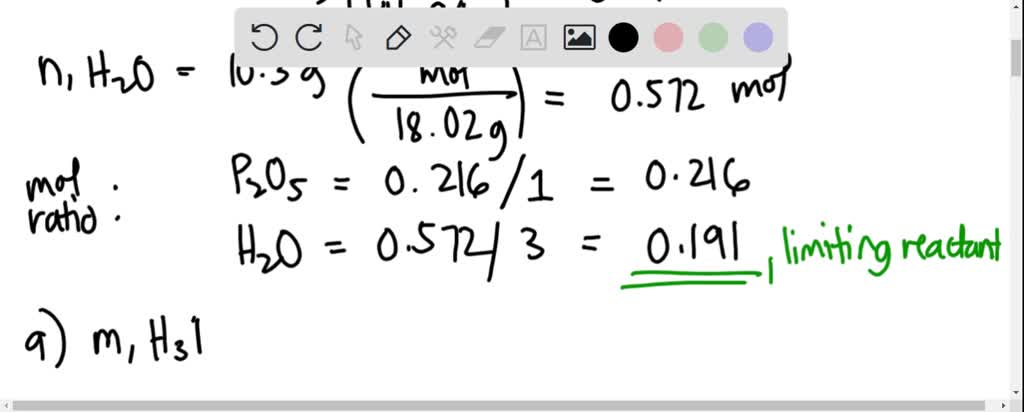
SOLVED: Identify the limiting reactant in the reaction of diphosphorus pentoxide and water to form H3PO4, if 27.4 g of P2O5 and 14.2 g of H2O are combined. Determine the amount (in

CCLXXXI.—The systems B2O3–SO3–H2O and B2O3–P2O5–H2O - Journal of the Chemical Society (Resumed) (RSC Publishing)
ntP2O5 on treatment with exces s of H2O followed by excess of NH4OH forms (NH4)2HPO4. If hundred gram of (NH4)2HPO4 is formed then find out the mass of p 2 o5 initial